

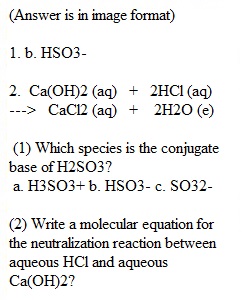
Q CHM 1032 Acid/Base Focus Concepts Name______________________ Period__________________ Date___________________ Directions: read each section and answer the questions accordingly. Show all mathematical work. (1) Which species is the conjugate base of H2SO3? a. H3SO3+ b. HSO3- c. SO32- (2) Write a molecular equation for the neutralization reaction between aqueous HCl and aqueous Ca(OH)2? (3) The titration of 20.0-mL of a sulfuric acid solution of unknown concentration requires 22.87-mL of a 0.158M KOH solution to reach the equivalence point. What is the concentration of the unknown sulfuric acid solution? Hint: write the balance molecular equation for the neutralization reaction. (4) Calculate the [OH-] in each solution and determine whether the solution is acidic, basic, or neutral a. [H3O+] = 7.5 x 10-5M b. [H3O+] = 1.5 x 10-9M c. [H3O+] = 1.0 x 10-7M (5) Calculate the pH of each solution and indicate whether the solution is acidic or basic. a. [H3O+] = 1.8 x 10-4M b. [H3O+] = 7.2 x 10-9M
View Related Questions